Cytotoxic T Cells — sometimes called the cancer assassins — belong to our armies of white blood cells. They specialize in recognizing and eliminating cancer cells and cells infected with viruses. Once they identify their target cells, there is no going back, only forward — cytotoxic T cells are serial killers. They hunt down, attack and then eliminate cancer cells and cells infected with viruses, one after the other, tirelessly.
To kill their targets, cytotoxic T cells use a mechanism called polarized secretion. They inject highly toxic, lethal proteins known as cytotoxins into their target cells, and they do so precisely and selectively — their lethal injection system spares the healthy, neighboring bystanders cells.
Now, a research team has captured the process on film, using state-of-the-art imaging techniques. The film — produced using movies from a study published a few days ago in the journal Immunity — shows cytotoxic T lymphocytes as they hunt down and eliminate cancer cells before moving on to their next target. Team leader Gillian Griffiths said in a press release: “In our bodies, where cells are packed together, it’s essential that the T cell focuses the lethal hit on its target, otherwise it will cause collateral damage to neighboring, healthy cells. Once the cytotoxins are injected into the cancer cells, its fate is sealed and we can watch as it withers and dies. The T cell then moves on, hungry to find another victim.”
In the film (shown below), a cytotoxic T cell finds a cancer cell (blue). Membrane protrusions from the cytotoxic T cells rapidly explore the surface of the cancer cell, checking for tell-tale signs that this is an uninvited guest. The cytotoxic T cell binds to the cancer cell and injects into it poisonous proteins known as cytotoxins (red).

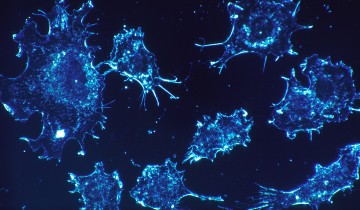

The article mentions the ability of Cytotoxic T Lymphocytes to attach to both cancer cells and virus-infected cells, and thereby release Cytotoxins into the cells perceived as a threat to the immune system in order to dispose of them. The 4D imaging technique mentioned in the article entitled “Actin Depletion Initiates Events Leading to Granule Secretion at the Immunological Synapse” illustrates the series of steps that Cytotoxic T cells takes, where it targets specific cells though an immunological synapse. Here, actin depletion is emphasized, which eventually yields centrosome polarization and secretion. This mechanism which is illustrated by the highly detailed and intricate video, is important as it has implications for both the development of viral strategies and cancer treatment.
Some Immunotherapy based treatments for cancer, demonstrate similarities with the mechanism exhibited by Cytotoxic T Lymphocytes. For example, Ipilimumab, a cancer drug designed to fight melanoma, relies on melanoma –specific Cytotoxic T-cells to attack melanoma cells, thereby avoiding or reducing collateral damage to nearby healthy tissue and cells.
In another study titled “Cytotoxic T Cell Adoptive Immunotherapy as a Treatment for Nasopharyngeal Carcinoma”, Epstein-Barr Virus is targeted with autologous Cytotoxic T cells, which are specifically created to interact with latent membrane proteins present in patients with Nasopharyngeal Carcinoma. Again, an emphasis was placed on avoiding collateral damage by avoiding damage to nearby healthy cells and tissues.
Work Cited:
Chambers CA, Kuhns MS, Egen JG, Allison JP (2001). “CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy”. Annual Review of Immunology 19: 565–94.doi:10.1146/annurev.immunol.19.1.565.
Hutajulu, Susanna Hilda, et al. “Therapeutic implications of Epstein–Barr virus infection for the treatment of nasopharyngeal carcinoma.” Therapeutics and clinical risk management 10 (2014): 721.
Lipson EJ, Drake CG (Nov 2011). “Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma”. Clinical Cancer Research 17 (22): 6958–62.doi:10.1158/1078-0432.CCR-11-1595.
Ritter et al., 2015, Immunity 42, 864–876. May 19, 2015 ª2015 Elsevier Inc.
http://dx.doi.org/10.1016/j.immuni.2015.04.013
Sondak VK, Smalley KS, Kudchadkar R, Grippon S, Kirkpatrick P (Jun 2011). “Ipilimumab”. Nature Reviews. Drug Discovery 10 (6): 411–2.doi:10.1038/nrd3463.
Thumar JR, Kluger HM (Dec 2010). “Ipilimumab: a promising immunotherapy for melanoma”. Oncology 24 (14): 1280–8.
Cytotoxic T lymphocytes belong to αβT-cells sub-population. They are very precise in killing the cancer and virus infected cells. They have CD8 co-receptor and can recognize MHC class I infected cells and kill them. Recognition of target cell by CTL leads to expression of FAS ligand which binds to FAS on target cell. This interaction signals release of highly toxic cytoplasmic granules, eventually activating killing pathway with the help of FAS/FASL interaction. They rearrange their micro-tubules and organelles in order to release the toxic granules by exocytosis. The granules contain granzymes (serine proteases), perforin and lysozymes. http://users.rcn.com/jkimball.ma.ultranet/BiologyPages/C/CTL.html. Most of CTL die after killing target cells but some become memory cells especially those which were helped by T helper cells.
Natural Killer cells play role in innate immune response, are another type of lymphocytes that also kill target cells but they are not T cells. Their killing mechanism is Antibody Dependent Cellular Cytotoxicity(ADCC), a non- phagocytic process. ADCC is triggered by binding of target bound antibody with Fc recepter on effector cells. http://www.els.net/WileyCDA/ElsArticle/refId-a0000498.html
References:
Alderson K. L., Sondel P. M. (2011). Clinical cancer therapy by NK cells via antibody-dependent cell-mediated cytotoxicity. J. Biomed. Biotechnol. 2011, 379123.10.1155/2011/379123
Seidel UJE, Schlegel P, Lang P. Natural Killer Cell Mediated Antibody-Dependent Cellular Cytotoxicity in Tumor Immunotherapy with Therapeutic Antibodies. Frontiers in Immunology. 2013;4:76. doi:10.3389/fimmu.2013.00076.
Cytotoxic T cells are types of white blood cells that kill damaged cells, infected cells, and specifically cancer cells. These cells can recognize specific antigens through their T-cell receptors (TCRs) that bind to the class I MHC molecule of the presenting cell. These TCRs recognize epitopes, small peptide fragments of antigens, displayed by MHC class I so that they can distinguish the difference between self-antigens and foreign antigens. This binding of the epitopes on the TCR involves a glycoprotein called CD8 to help recognize MHC classes and induce a response. Cytotoxic T cells use their receptors highly to hunt down their targets and kill them through polarized secretions. The cytotoxins they secrete rely on the use of microtubules in the cells to travel to their target cancer cells and kill them.
Cancer patients with tumors have a heightened survival rate when their cytotoxic T lymphocytes are infiltrated. The best way to eliminate cancer through an immunotherapy approach is through these specific T cells in numbers. The recruitment and infiltration to the site of tumor is the focus. T cell trafficking must be done to the specific tissue. Chemokines, signaling proteins, help with the recruitment of these lymphocytes and play a major role in trafficking the cells during activation. Along with the number of tumor-infiltrating T cells, they also must be antigen-specific. The T cells need to be able to recognize the antigen that shown by the MHC to enhance the migration and exert their potentials. There are studies known to help with T- cell infiltration by increased cytokines like IFN-γ and chemokines. Ipilumimab was previously mentioned in a comment and has shown great results. Further investigating, there is also a study shown that ipilumimab, a cytotoxic T lymphocyte antigen-4 blocking antibody, also can improve T cell infiltration to the tumor tissue as well as help with their survival. VEGF (Vascular endothelial growth factor) blockers and ipilumimab have already been approved as first line therapies. The study wanted further investigation on the actual mechanism of T cell infiltration with chemotherapies as well as anti-angiogenic inhibitors. If these methods of infiltration is studied more then hopefully in the future there can be a better solution for cancer immunotherapy.
Oelkrug, C., & Ramage, J. M. (2014). Enhancement of T cell recruitment and infiltration into tumours. Clinical & Experimental Immunology, 178(1), 1-8. doi:10.1111/cei.12382
Great point Leanna! I too agree that the most important objective of cancer immunotherapy should be increasing the numbers of cytotoxic T cells that are recruited to the area of infection in order to attack and defeat the cancer cells. In order for this to be achieved, therapy must focus more on the activity and signaling of the cytokines like IFN-y as you mentioned. If there is a lack of cytokines to recruit the cytotoxic T cells then elimination of the cancer cells becomes much more difficult. However, when plenty of cytokines are available, more recruitment is possible and cancer cells will be much more likely to lose the battle. Additionally, further knowledge must be conducted on the manner that cancer cells inhibit or suppress supporting cytotoxic T cells. This could lead to discoveries that free up more CD8 cells which will ultimately lead to higher rates of tumor and cancer suppression.
Cytotoxic T lymphocytes are major components of adaptive immunity, which play an important role in the scavenging the infectious pathogens and tumor cells. An effector cytotoxic T lymphocytes has the ability to recognize the target cells with the help of active TCR and peptide fragments on the cell surface presented by the MHC class I molecules. They also promote the apoptosis of the cells through a combined mediated mechanism of granules (perforin/granzyme) – and receptor (tumor necrosis factor).
So the cytotoxic T lymphocytes can be deployed for antitumor immunity. Apart from their cytotoxic nature there are some other factors which enable them to be considered for antitumor medication.
1. The widespread expression of the MHC class I, allows the cytotoxic T cells to be easily deployed for tumors from diverse origin.
2. Cytotoxic T lymphocytes are continuously rearticulated in the blood to find the antigens.
3. The selection of targets is highly sensitive and precise.
4. Cytotoxic cells also triggers the production of INF-γ, a cytokine with several antitumor
properties.
Notably Cytotoxic T cells have been used in development of vaccine for some virus mediated malignancies. A recent extended approach, is development of tumor cell based vaccine engineered to express immunomodulatory cytokines and costimulatory molecules. A major difficulty faced in this type of vaccine is in vitro monitoring. Similarly approach in development of non-viral tumor vaccines are based on molecular targeting. Mostly the antigens from over expressed tumor cells are used. The close relationship between tumor antigens and self-antigens remains an obstacle here from using these as a successful vaccine against tumors.
However, more studies and development of new strategies, along with the efficient cytotoxic T lymphocytes will definitely provide a successful vaccine for tumor in near future.
References:
J Maher, E T Davies,2004, Targeting cytotoxic T lymphocytes for cancer immunotherapy, British Journal of Cancer. 2004 Aug 31; 91(5): 817–821.
Electron micrograph images showing cytotoxic T cells (CTLs) aligned with cancer cells as they release perforin and granzymes directly into them are striking. Knowing that they do so in a manner designed to preserve surrounding, healthy cells leads one to posit the work of divinity. However, seeing such a beautiful immunofluorescence film that captures the entire process of T cell migration to cytotoxic secretion at the immunologic synapse took my breath away.
Having worked with DAPI, GFP and RFP tagging for immunofluorescence imaging, I appreciate how difficult the process is to capture a single, clear image of intracellular structures. While reading the study by Ritter et al. (http://www.cell.com/immunity/abstract/S1074-7613%2815%2900173-9), I was impressed with the creativity that went into designing an experiment that would not only map out the sequence by which CTLs target and destroy cancer cells, but also provide high resolution 4D imaging that clearly depicts each step. Watching as the CTLs approached their cellular targets with the actin-rich projections at the front side of their amoeba-like movements, then as rapid actin depletion triggered TCR and cSMAC clustering followed by centrosome polarization, I had to remind myself that I was not watching an animal on the hunt, but a single immune cell on a mission. The docking and polarization of the granules occurred in less than ten minutes, but the work that went into capturing the images on lattice light sheets and then linking them to create the film must have taken months. What an amazing production!
I watched the video above at least five times and I continue to have the same recurring thought. The human body is amazing. More specifically, the immune system is a work of art. Cytotoxic T cells are the one type of serial killer that I don’t mind having around. The way they tenaciously patrol for invaders is a key part of why we as humans are capable of being healthy. The video explains well that these cells are needed for a fight against cancer cells. Unfortunately, T regulatory cells are often recruited by cancer cells which protect it from destruction by cytotoxic T cells. I look forward to reading studies that show natural ways to “dispose” of T regs so that cytotoxic T cells may be able to do their job in the serial killing of cancer.
Works cited:
Nahas GR, Walker ND, Bryan M, Rameshwar P.A Perspective of Immunotherapy for Breast Cancer: Lessons Learned and Forward Directions for All Cancers. Breast Cancer . 2015 Nov 2;9(Suppl 2):35-43.
The function of cytotoxic t cells isn’t just to destroy cancer cells. Cytotoxic T cells are also important in destroying virus-infected cells. In both cases, the cytotoxic T cell uses a system of delivering cell destroying granules specifically to the cell in question, thus reducing the bystander effect and allowing nearby cells that are not infected to live.
Cytotoxic T cells are part of the adaptive immune response, which is key for generating memory T cells, thus preventing future infection of the same viral pathogens. Cytotoxic T cells can also play a role in autoimmune disorders such as type I diabetes and lupus. It’s so interesting to see how one cell type can have a variety of effects in the body and how upregulation or downregulation of a particular cell type or associated molecules can result influence those effects.
http://www.nature.com/jid/journal/v126/n1/abs/5700001a.html
http://www.ncbi.nlm.nih.gov/pubmed/16224251
As the video mentioned, Cytotoxic T-cells absolutely play a role in the destruction of cancer cells, and as Torellas points out, Regulatory T-cells can play a role in the longevity of cancer progression.
Torellas makes a very interesting point in regards to the role of Regulatory T-cells, cytotoxic T-cells, and fighting cancer cells. In a study conducted by Dr. Qiang Qao, titled “Intratumoral Balance of Regulatory and Cytotoxic T Cells Is Associated With Prognosis of Hepatocellular Carcinoma After Resection “, the efficacy of Regulatory T-cells in hepatocellular carcinoma is surveyed. 302 patients were examined with factors including CD3+, CD4+, CD8+, Foxp3-positive, and Regulatory T-cells were analyzed by immunochemistry in tissue microarrays.
The results from this study illustrate several main points. First, CD3+, CD4+, CD8+, and Regulatory T-cells were not indicative of overall survival nor disease-free survival. The presence of low intratumoral Regulatory T-cells, matched by a high concentration of activated CD8+ cells was directly linked with increased overall survival rates and disease-free survival rates, which bolsters Torellas’s point. This can serve as an effective form of immunotherapy, which can decrease recurrence and lengthen survival following surgical treatment.
While these results are telling, further research needs to be conducted in order to draw a more decisive conclusion.
Work Cited:
Gao, Qiang, et al. “Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection.” Journal of Clinical Oncology 25.18 (2007): 2586-2593.
T reg is one of the many strategies tumor tissue uses to live and expand in human body. The general idea is that tumor cells camouflage themselves with cell surface proteins and secreted cytokines/other factors as a wounded tissue, and demand the immune system to support with their “tissue repair.” T reg is highly involved in the normal wound healing process and thus recruited by tumor cells to suppress multiple killing mechanisms used by the immune system, including apoptosis and necrosis induced by T killer cells, NK cells, neutrophils and phagocytosis by macrophages. The immunotherapies are now mainly focusing on either enhancing the killing functions or inhibiting the regulatory functions, which is provided by T reg, MDSC, tumor associated macrophages.
Cancer is very manipulative. Using one T-regulatory cells for is regulations is very smart because it keeps it under the radar from Cytotoxic T-Cells. Yet sometimes the Cytotoxic T-Cells still ends up killing cancer cells like we see in the video. It makes one wonder cancer cells blow their cover and gets killed by Cytotoxic T cells? or Cytotoxic cells are just that good at detecting the manipulative cancer cells? I really admire the Cytotoxic T-cells because they are very hard working and without them one would suffer from a whole lot of infections and disease.
I agree with you that the human body is amazing, the more I learn in my graduate studies, the more fascinated I am by the complexity of the human body. Cytotoxic T-cells are primarily responsible for killing cancer cells, other infected cells, or cells damaged in other ways. It is unfortunate that T regulatory cells are recruited by cancer cells to protect cancer cells from destruction by cytotoxic T cells. A recent study shows that modified cells called super natural killer cells can attack cancer cells and prevent metastasis by attaching a TRAIL protein and E-selectin adhesion receptor to the natural killer cells. It would be interesting to see if cytotoxic T-cells could also be modified in a similar way to attack cancer cells protected by T reg cells.
doi: 10.1073/pnas.1316312111
The role that cytotoxic T cells as well as other immune cells play in autoimmune disorders such as lupus is intriguing. Systemic lupus erythematosus (SLE) is a disease that adversely affects the immune system. T cells of patients with SLE express less interleukin than healthy individuals. There is a faster and stronger signaling response in SLE T cells to calcium influx that leads to less production of IL-2. Patients with SLE express lower levels of CD3, which could be due to a number of factors such as decreased transcription, decreased stability of mRNA, decreased protein degradation and alternative splicing. The cytotoxic activity of SLE patients is significantly decreased. The inability of SLE patients to kill infected cells may lead to autoantibody production. SLE patients also exhibit lower regulatory T cells than normal. SLE T cells express higher levels of a certain surface adhesion molecule. SLE T cells also show stronger adhesion as well as faster migration in response to chemokines than normal. SLE displays a host of T-cell abnormalities that include decreased cytotoxic activity of CD8 T cells.
I completely agree with previous comments about the difficulty of acquiring good images of single fluorescently tagged cells in vivo. The fact that there are entire papers dedicated to gathering higher resolution images of single cells [1] just lets you know this is the undertaking of a lifetime. I have been the victim of countless struggles trying to capture the perfect image at the perfect moment in an effort to prove to the scientific community that the results you are reporting are actually accurate. To see such a beautiful rendition of a process that is happening countless times a day in your body puts my efforts and current imaging techniques to shame.
The one objection I have to this video is the name. While I understand the appeal and intrigue of calling a cytotoxic T cell a “serial killer”, I think it detracts from the wonder of the cell’s function. From the standpoint of a person who has spent time doing science outreach for smaller children and the non-scientific community in general, people are generally put off by small details like calling a cell a “serial killer”. It leads to people assuming that their bodies could at any point in time turn against them and make them sick when in reality it is a normal, every day function that ensures their survival. While to scientists it’s just a fun buzzword to include in their studies, it has ben shown that how you phrase the titles [2] of articles, pictures and goods biases the reader towards a specific way of thinking, depending on their past experiences with the word [3].
While it seems like a trivial thing to us, the non-scientific community plays a large role in the studies we conduct (I think we can all remember the debacle about stem cell use in research) and phrasing concepts in manners that appeal the public might not only be a good idea, but a necessary evil.
[1] Kang M., et al. Live imaging, identifying, and tracking single cells in complex populations in vivo and ex vivo. Methods Mol Biol. 2013;1052:109-23. doi: 10.1007/7651_2013_19.
[2] Wiley, J.; Rayner, K. Effects of titles on the processing of text and lexically ambiguous words: Evidence from eye movements. Memory & Cognition
November 2000, Volume 28, Issue 6, pp 1011-1021 doi: 10.3758/BF03209349
[3] Fagerlind, H., et al. Patients’ understanding of the concepts of health and quality of life. Patient Education and Counseling. Volume 78, Issue 1, January 2010, Pages 104–110. doi:10.1016/j.pec.2009.05.016
The blog post discusses the importance of CD8+ T cells in controlling cancer cells. However, CD8+ T cells are also very important for controlling chronic viral infections. CD8+ T cells recognize infected cells though MHC class I molecules and lyse the cells by secreting degranulating proteins. Viral infections that cannot be controlled, such as HIV infections, do so by mutating to evade the CD8+ T cell response. HIV also insidiously alters CD8+ T cell signaling, causing there to be less effector and memory CD8+ Tcells that can take care of the infection. This shows how important CD8+ T cells are. Not only do they help prevent cancer, but viral infections must find ways to circumvent their actions to succeed.
Source:
Gulzar N, Copeland KF. CD8+ T-cells: function and response to HIV infection. Current HIV Research. 2004 Jan; 2(1):23-37.
This individual discussed the overall function of CD8+ T cells as they kill cancer cells and cells infected with viruses. If not already, I think it is important to conduct experiments the identify which cancers are more prone to dysfunction of CD8+ T cells and to identify if there is correlation between the amount of CD8+ T cells in an individual and the stage of their cancer.
As a major part of the adaptive immune system, T-cells scan the intracellular environment in order to target and destroy infected cells. They not only kill cancer cells and virus infected cells but other cells which are damaged in other way. It is very important to understand the mechanism of Cytotoxic T Lymphocytes antigen recognition and activation to better understanding and treatment of diseases. They play role in transplant rejection. http://www.tcells.org/scientific/killer/
Recently platelets have been shown to facilitate the accumulation of virus-specific cytotoxic T cells into the infected liver. Recently, cytotoxic T cells have been implicated in the progression of arthritis: depletion of knee joint cartilage macromolecules such as glycosaminoglycans by cytotoxic T cells and macrophages has been observed in a rat model of the disease. https://en.wikipedia.org/wiki/Cytotoxic_T_cell
Through IL-10, adenosine, and other molecules secreted by regulatory T cells, the CD8+ cells can be inactivated to an anergic state, which prevents autoimmune diseases. CTL are induced by several diverse stimuli, including major histocompatibility antigens, protein antigens, viruses, and intracellular bacteria and parasites.
Ito H, Seishima M. Regulation of the induction and function of cytotoxic T lymphocytes by natural killer T cell. J Biomed Biotechnol. 2010;2010:641757. doi:10.1155/2010/641757.
I agree, the CD8 T cells are important to other infections, such as, viruses, as well. The cells are involved in killing other viruses that invade the body. Cytotoxic T cells are the cells running the show, since they are capable of producing lethal secretions to assassinate invaders. I think more research is necessary in understanding the specificity of the cytotoxic T cells and to develop subgroups and investigate their functions. The more we understand about the pathways, the development, and the functions of these cells, we can better understand ways of targeting or assisting the cells through therapies and pharmaceuticals.
This technology shows and confirms that cytotoxic T-cell kill cancer cells by using cytotoxins with the direction of the microtubule organizing center (MTOC), but what happens when cancer cells evade this mechanism and turn into malignant neoplasms that metastasize? Research has shown that tumor cells can be resistant to cell death and can develop mechanisms to retaliate against the immune response (5). Cancer cells on occasion are ignored by the immune system failing to activate the APC’s (5) such as dendritic cells to go to the secondary lymphoid organs to active CD8+ T-cells. Mutations can occur that down regulate tumor antigens production causing the peptide to not be presented by MHC class I to the TCR on CD8+ T-lymphocytes therefore failing to activate of CD8+ T-lymphocytes to release cytoxins (5). In addition, cancer cells are resistant to CD95L a ligand expressed on cytotoxic T-lymphocytes upon cell contact that causes cell death in cancer cells through the activation of death-inducing signaling complex (DISC) containing CD95, FADD Fas-associated with a death domain (FADD), procaspase-8 &10 and caspase-8 &10 (1). Lastly, most tumor cells express CD95 and CD95L and use it as “counterattack” on cytotoxic T-lymphocytes (2, 3, 4)
1. Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ 2003; 10: 26–35. |
2. Igney FH, Behrens CK, Krammer PH. Tumor counterattack—concept and reality. Eur J Immunol 2000; 30: 725–731.
3. Chappell DB, Restifo NP. T cell-tumor cell: a fatal interaction? Cancer Immunol Immunother 1998; 47: 65–71.
4. O’Connell J, O’Sullivan GC, Collins JK, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996; 184: 1075–1082.
5. Igney, F.H. & Krammer, P.H. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002 Jun;71(6):907-20.
It’s beautiful to watch the interplay between Cytotoxic T Cells and their targets. And the techniques involved to gather such evidence is amazing. I can’t even imagine the hours and effort it must have taken to make this video!
Incredibly this process is far from static, as each participates in coevolution over time, responding back and forth for survival. It’s difficult not to anthropomorphize these exchanges as battles whereby each side is constantly escalating their defenses/offenses in a virtual molecular arms race. Many studies have shown that as pathogens and even cancer cells evolve to escape leukocytes (and in particular lymphocytes such as CTLs), the immune system also evolves to keep up with its adaptive immune response where specificity is achieved through alternative splicing and somatic recombination (Hertz et al., 2011).
Hertz, T., Nolan, D., James, I., John, M., Gaudieri, S., Phillips, E., & … Jojic, N. (2011). Mapping the Landscape of Host-Pathogen Coevolution: HLA Class I Binding and Its Relationship with Evolutionary Conservation in Human and Viral Proteins. Journal Of Virology, 85(3), 1310-1321.
Unfortunately, the cancer cells that are eventually able to develop into malignant cancers have multiple ways to damage the immune system and survive from the attack. Normally they are able to cripple the function of killer cells by secreting immuno-inhibitory molecules such as TGF-β and PEG2, or induce the generation of immuno-inhibitory cells such as Tregs or MDSCs. Some of these cancer cells are even able to kill the engaging T cells through the Fas-FasL pathway. The cancer cells may also impair the function of DCs, retarding their maturation, inhibiting their ability of antigen processing and presentation. The cancer cells may stop expressing the cancer antigen recognized by the immune system, and of course they don’t have that much of MHCⅠ and co-stimulatory molecules on the surface as the normal cells do. In short, the loss of immunogenicity of the cancer cells are the main reason of their escaping from the immune attack, and the idea of immunotherapy has become a hot spot in Cancer Therapy. However, the most evidently effective ways to treat cancer for now are still surgical removal, cytotoxic chemotherapy and radiotherapy.
Cytotoxic T cells play a very key role in the immune system. They are known to kill infected cells as well as cancerous cells. After watching the video it amazes me how they are able to detect and kill cancerous cells. They also do it without harming neighboring cells and this precision is very remarkable.
If Cytotoxic T cells are capable of eliminating cancer cells, how come cancer still exists? If more study is done on these T cells, I believe cancer can be prevented and also fully developed cancer cells can be reduced as well. When tumor specific Cytotoxic T-lymphocytes where induced in cancer patients, the tumor cells reduced due to the cytotoxic t cell ability to kill cancer cells.
I propose more studies to be done on Cytotoxic T cells because it can potentially lead development of new cancer therapies.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1422470/
You bring up a good point. If these cytotoxic cells have the ability to eliminate cancer cells, why does it still exist? Through this blog and various other studies, we already know the extreme significance the cytotoxic T cells play in our bodies. And they do so with a very efficient process. Current research on cancers focuses on the functions of say anti-antibodies. Studies have found that in mice, if we use a monoclonal antibody specific to CD47 (protein on cancer cells) to block its activity, we see that it reduces tumor growth by enabling macrophages to phagocytose the target cells. CD47 protein can be looked upon as ones that give the “don’t eat me” signal. The expression of it on the cancer cells tells the macrophage not to eat it. Now why is it that the anti-CD47 is important and how is it relevant to this post? Well, results have shown that the anti-antibody results in the activation of CD8 cytotoxic T cells, which go on to kill the tumor cells efficiently as described in this blog post. Once the anti-CD47 (monoclonal antibody) enables phagocytosis, the macrophage presents to the T-cell peptides from the cancer cells and stimulates CD8 cytotoxic T cells, which go on to kill the cancer cells. Science is constantly evolving and we are learning new things every day. This study can be looked upon as a potential landmark, providing a potential key in finding a solution or better treatment therapies for cancer.
References:
Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142(5):699-713. doi:10.1016/j.cell.2010.07.044.
Unanue ER. Perspectives on anti-CD47 antibody treatment for experimental cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(27):10886-10887. doi:10.1073/pnas.1308463110.
As I sit and watch this video, I’m constantly amazed at the effective functions our immune system can facilitate in order to keep us healthy. Now this is a very interesting mechanism, I believe. These cytotoxic T cells release granule contents which accounts for majority of the cytotoxicity activity. This granule-mediated killing, however, is strictly calcium dependent and a study finds that some cytotoxic t cells survive without it. So there has got to be another mechanism of cytotoxicity as well in addition to the granule-mediated mechanism. So another mechanism involves binding Fas in cancer cell by the Fas ligand, which will induce apoptosis. The study additionally finds that these interactions are important in order to terminate lymphocyte growth after the pathogen has been removed. That being said, I think it is completely fascinating at how effectively our immune cells/system is able to clear infections (in this case, cancer cells). If one way does not work, we have a potential “back up plan” ready.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001. T cell-mediated cytotoxicity. Available from: http://www.ncbi.nlm.nih.gov/books/NBK27101/
Cytotoxic T cells are the vindictive cells of the immune system. They are known for their capability to kill certain cells, including foreign, viral, and cancer cells. These cells could be a great basis for developing therapeutic measures for patients suffering from diseases or illnesses, such as, HIV or cancer, since these are known to attack the immune system. The body lacks the ability to create these white blood cells when it is attacked by immune suppressing illnesses. Therefore, the cells could be cultured and sub-cultured (grown in a laboratory) and used as a therapy. Induction of CD8+ CTLs that are specific for tumor antigens is a good way of developing a basis for new cancer vaccines. CTLs are highly specific and mediate the death of cancer cells, even at low antigen expression on the target cells. (Hariharan, 1995) These cells are quite amazing and show that they may be one of the most vital cells considering the countless cancer cells or other foreign cells that invade the human body.
Works Cited
Hariharan, K. B. (1995). The Induction of Cytotoxic T Cells and Tumor Regression by Soluble Antigen Formulation. Cancer Research , 55, 3486-3489.
Cytotoxic T cells are the immune cells responsible for eliminating cancer cells and viruses. The Cytotoxic T cell has a T cell receptor with a CD8 co-receptor that recognizes MHCI receptors on a cell surface. Upon attachment, Cytotoxic t cells are able to secrete its cytotoxins into their target cell inducing cell death, and are able to move on the next cell. Therefore, labeling Cytotoxic T cells as “serial killers” are appropriate, given the mechanism involved with eliminating cancer cells and virus.
The effector functions of a cytotoxic T cell is determined by the different molecules that it produces. The effector molecules that are produced by cytotoxic T cells may be either a cytotoxin or a cytokine. The former is released by a CD8 T cells and stored in granules while the latter is a membrane-associated protein that is synthesized by all effector T cells. Cytotoxins are the prominent effector molecules of cytotoxic T cells; these T cells possess the ability to use their membrane protrusions to recognize characteristics of an invading pathogen and then inject its cytotoxins. This interaction between the cytotoxic T cell and its target is initiated by brief non-specific adhesion mechanisms between the two cells. The recognition of the target produces a tighter and stronger interaction as opposed to the initial transient interaction. This stronger interaction leads to the release of cytotoxins to the target that may activate an intrinsic death programming. Because the release of cytotoxins is not specific, their release must be regulated tightly. Their release must be tightly regulated because of the fact that they can penetrate the lipid bilayer. Once a cytotoxic cell releases cytotoxins that penetrate a cells lipid layer, it has the ability to activate apoptosis. A CD8 T cell expresses a Fas ligand which is the main TNF-related molecule which cytotoxic T cells express that is membrane associated and induces programmed cell death in cells that contain the Fas receptor protein. Lymphoproliferative diseases are similar to cancer due to their association with autoimmunity. When the mechanism of CD8 T cells, expressing a Fas, that induces cell death in a cell that contains an Fas receptor protein is not activated, lymphoproliferative disease occurs.
(http://www.ncbi.nlm.nih.gov/books/NBK27149/)