The skin is the largest organ of our body — in terms of both weight and surface area. One of its many functions is to provide a protective barrier between the external and internal environment. However, it is more than a physical barrier: it is an active immune organ.
Skin defenses are organized around a complex regulatory network that includes not only cellular, but also microbial components. Indeed, the skin is an ecosystem composed of diverse habitats — folds, invaginations and specialized niches that support a large variety of microorganisms, the skin microbiota. The skin is also home to different cell types. While cells such as macrophages, dendritic cells and mast cells have long been recognized as members of the skin defense network, recent independent breakthroughs reveal the participation of other cell types — notably memory T lymphocytes and adipocytes.
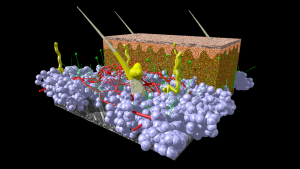
A model of human skin. Fat cells from the hypodermis are given in blue. Blood vessels in red, nerve tissue in green and hairs, associated glands and other glands in yellow. Connective tissue of hypodermis and dermis is given in brown. The epidermis on top is given in red/pink color. Image credit: Scivit, CC BY-SA 4.0
Recently, results from a study carried out by a team of scientist at the Brigham and Women’s Hospital in Boston demonstrated that the human skin is protected by four functionally distinct types of memory T cells, which include two populations of resident T cells and two populations of recirculating T cells. Although the presence in mouse and human skin of both resident and recirculating memory T cells was already known, this recent study advances our understanding of skin defenses by providing novel information on the physical distribution of the four distinct subpopulations, their relative proportions and their functional activities. Each subpopulation is likely to play a unique role in protecting against infectious pathogens — and not only. Both resident and recirculating memory T cells are known to contribute to human diseases of the skin. For example, psoriasis is linked to dysregulated resident memory T cells, whereas leukemic variants of cutaneous T cell lymphoma represent a malignancy of the recirculating cells.
The discovery of different subpopulations of resident and recirculating memory T cells highlights the complexity of the skin defense network. Such complexity is augmented by the contribution of a cell type that, until recently, was not a known member of the network: the adipocyte. Adipocytes are cells specialized in storing energy as fat — in other words, they’re the so-called fat cells.
The skin consists of two layers – the outermost epidermis, and the underlying dermis. Beneath the dermis lies the subcutaneous adipose tissue, which is mostly made up of adipocytes. Not too long ago, Richard Gallo and collaborators noticed that infections by methicillin-resistant Staphylococcus aureus (MRSA) resulted in a considerable enlargement of the mouse dermal adipose tissue as a consequence of an increase in both size and number of adipocytes.
They found that two adipogenesis-inducing transcription factors, zinc finger protein 423 (ZFP423) and peroxisome proliferator–activated receptor γ (PPARγ), mediated the increase in adipose tissue. Inhibition of these transcription factors, and therefore inhibition of adipogenesis (the process by which pre-adipocytes differentiate to adipocytes), resulted in increased severity of skin infection, indicating a possible adipocyte role in promoting host defense against MRSA.
How do adipocytes, then, participate in host defense? The researchers discovered that differentiating adipocytes — in response to infection — secrete cathelicidin, which can directly kill bacteria. Cathelicidin is an antimicrobial peptide produced mostly by macrophages and neutrophils — the ability of adipocytes to secrete antimicrobial peptides came as a surprise. Interestingly, mature adipocytes produced less cathelicidin. Cathelicidin-deficient mice exhibited increased bacterial growth, whereas inhibition of adipogenesis in these mice did not result in a more severe infection. Thus, cathelicidin is the main player in the host defense mediated by adipocytes.
How can these recent discoveries guide development of novel therapeutic approaches? At this point, we are in the realm of speculation. The characterization of memory T cells subpopulations at the level of phenotypic and functional properties could lead to their selective and differential targeting for autoimmune and inflammatory diseases of the skin. Indeed, the current therapeutic modalities for these disorders do not take into account the involvement of the relevant memory T cells. Similarly, it is reasonable to expect that the increased understanding of the skin memory T cell system will influence the development of novel vaccination strategies that induce, when needed, generation of the appropriate memory T cell subpopulations.
Could adipocytes be the target of therapeutic approaches designed to treat MRSA infections? Conditions that hinder pre-adipocyte functions can predispose to infection. Thus, one approach could be to restore these functions, even using adipocyte stem cell therapy. What about augmenting cathelicidin production by normal adipocytes during infection? Adipocytes express a variety of toll-like receptors (TLRs), and the sensing of S. aureus by adipocytes might be TLR-mediated, most likely by TLR2. Thus, it has been proposed that PPARγ agonists could be pharmacologically used to control cathelicidin production by targeting the TLR2-ZFP423-PPARγ -cathelicidin pathway. An additional potential therapeutic approach could be based on vitamin D. Indeed, 1,25-Dihydroxyvitamin D3, the active form of vitamin D, is a major regulator of cathelicidin expression in monocytes and in epidermal keratinocytes, and is also known to modulate adipocyte function. It could be worth to determine whether or not vitamin D regulates cathelicidin expression in adipocytes, and explore the possibility of using vitamin D to support the current therapeutic modalities for MRSA infections.
These proposed approaches seem logical. However, in the airways, S. aureus exploits cathelicidin to promote staphylokinase-dependent fibrinolysis, leading to enhanced bacterial dissemination and invasive infection. Although the same pathogenic mechanism may not be operating in the skin, development of the proposed therapeutic approaches will need to take into account the potential unwanted interaction of S. aureus with cathelicidin.
The exciting discovery of unexpected players that may contribute to protective responses will guide, in the next few years, the design of novel therapeutic strategies that selectively target specific cell types — to effectively control infections and autoimmune/inflammatory diseases of the skin.



The skin is most often seen as the simplest explanation to immune system response. In fact, I am sure that every person who has ever taken an immunology course (like the one my classmates in BIOL 6278 are in now) is sure to remember some sort of picture or slide of a pathogen entering broken skin and activating the host’s immune response. Sure, to us it seems simple and straightforward, but as this article explains, the role of skin in immune defense is far greater than just a physical barricade against infection. It is a series of cell populations that develop the ability, over time, to respond to common threats to the host, and in doing so, protecting its overall health.
The fact that there are new discoveries about different cell lineages working, in some way or another to promote immune defense, further punctuates the fact that each organ system molds seamlessly into the other and there is no real way of separating where the work of one ends and the other begins because they mostly come from similar progenitor cells that differentiate into several lineages by the presence of cytokines, growth factors and other forces in the microenvironment[1]. The fact that adipocytes can create certain molecules that can kill bacteria seems to me a type of bacterial containment strategy. Because adipocytes are mostly located under the dermis [2], an increased adipocyte production (because young cells create more cathelicidin) mimics the effect of inflammation of the skin in keeping pathogens concentrated in one area and keeping them from entering circulation while sentinel cells clear the infection and call on neutrophils.
As the author of this post mentioned, the skin is the largest organ in the body, and in being such, it should have a large role in protecting organisms from outside infections. To put it in more colorful terms, the skin is the high walls and moat surrounding a castle to keep invaders outside of its limits. The people defending these walls must be able to recognize enemies in order to prevent attacks, so the fact that many memory T cell populations exist within the skin does not surprise me in the least. However, it does open a good discussion about other types of defenses the skin might offer that we were not aware of[3], as well as open doors for new therapeutics. Not only may this help, as discussed in the blog and paper, the treatment of autoimmune skin diseases, but it may also open new avenues of thinking in regards to how we protect ourselves from every day infections.
[1] Bryant, D.; Mostov, K. From cells to organs: building polarized tissue. Nature Reviews Molecular Cell Biology 9, 887-901 (November 2008) | doi:10.1038/nrm2523
[2] Hausman, et al. Adipocyte development in the rat hypodermis. American Journal of Anatomy. Volume 161, Issue 1, pages 85–100, May 1981. Article first published online: 3 FEB 2005 DOI: 10.1002/aja.1001610107
[3] Pasparakis, et al. Mechanisms regulating skin immunity and inflammation. Nature Reviews Immunology 14, 289–301 (2014) doi:10.1038/nri3646
I believe the most intriguing discovery here is the immunological responsibilities of the adipocyte. Traditionally when we think of fat cells, we simply consider their simplest definition of storing fat as the cell’s sole function. As the researchers noted, the ability for adipocytes to secrete cathelicidin is a significant step in destroying early infections. With adipocytes being poised to be on the front lines after wall of skin has broken, flooding the body with external microflora, the ability of these cells to release such a powerful antimicrobial peptide is essential in assisting the bodies neutrophils. As we continue to learn more about the roles of adipocytes in regards to their immunological capabilities rather than just fat storage, I would be intrigued to see how the general public’s perception of fat cells will change. Will “fat-burning” dietary supplements now be restricted to those with healthy immune systems, or will there be a radical faction along the same vein of the anti-vaccine community insisting that oversaturated adipocytes lead to better overall health?
The addition of this new immunological role to the multifunctional adipocytes will definitely make people see adipocytes in a new light.
Adipose tissues from the obese subjects have been known to have a higher density of inflammatory macrophages, CD4+ T cells and fewer regulatory T cells. Which results in increased amount of inflammatory responses leading to cardiometabolic diseases. With the new immunological role, the interesting question will be:
What will be the proportion of the adipose tissue’s contribution to cardiometabolic diseases and the prevention of pathogenic diseases?
I think very good points were made as to weighing the protective function adipocytes can provide in support of the immune system versus the cardiovascular and chronic inflammatory conditions that may be worsened by visceral fat. However, the fact that different forms adipocyte tissue exist and the protective and pathogenic effects fat are more closely associate with particular types of tissue may be a way to cheat this dilemma.
The studies thus far have only demonstrated an immunological support role for subcutaneous fat and its distinction from the more dangerous visceral fat in everything from histology and tissue-specific microenvironment, to it’s reduced propensity for inducing metabolic disorders and chronic inflammation. Indeed, not only is excessive secretion of IL-6 mostly a visceral fat problem, but also it appears that subcutaneous fat does not appear to produce the risks associated with visceral fat.
If so, the choice has become easier and therapeutic, and dietary intervention should seek to increase the ratio of protective subcutaneous fat relative to visceral fat.
Bibliography: 1. Fontana L, Eagon J, Trujillo M, Scherer P, Klein S. Visceral Fat Adipokine Secretion Is Associated With Systemic Inflammation in Obese Humans. Diabetes. 2007;56(4):1010-1013. doi:10.2337/db06-1656.
Bibliography: 2. Lu Q, Li M, Zou Y, Cao T. Induction of adipocyte hyperplasia in subcutaneous fat depot alleviated type 2 diabetes symptoms in obese mice. Obesity. 2014;22(7):1623-1631. doi:10.1002/oby.20705.
Given the complexity of the immune system and respective cytokine network, I believe we will find that there is a significantly more intricate relationship between adipose and leukocytes. A study published in 2012 indicates a strong linkage between adipose tissue and macrophage differentiation, with obesity resulting in a significant leukocyte imbalance. The research suggests that obesity induced leukocytosis is the result of an imbalance between M1 and M2 macrophages. M1 Macrophages are more prevalent in obese adipose tissues, and are responsible for the release of inflammatory cytokines such as TNF, and IL-6. M2 macrophages are more prevalent in lean adipose tissues, and are in direct opposition to the M1 macrophages, releasing anti-inflammatory cytokines such as IL-10. This relationship leads me to theorize that all adipocytes play a larger role in leukocytic differentiation resulting in a network much more complex than previously thought.
Reference List
Kanneganti T, Dixit V. Immunological complications of obesity. Nature Immunology [serial online]. July 19, 2012;13(8):707-712. Available from: MEDLINE with Full Text, Ipswich, MA. Accessed November 30, 2015.
Vitamin D is again the missing link
Cytokines, T cells, cath, adipose tissue etc etc etc et al.
The focus must be on vitamin D’s role. Nothing regulates the genome as D metabolites.
I also find this new characterization of adipocytes as contributors to immune defense very interesting but I wonder at its overall effectiveness in fighting pathogens such as S. aureus. Once the infection has breached into the fat layer medical intervention in the form of antibiotics and drainage is generally required. However the results of the study you present are quite significant and could potentially bring about novel therapeutic strategies by increasing the secretion of cathelicidin. There are currently a family of drugs called thiazolidinediones on the market used to treat type 2 diabetes which increase production of PPARγ (Phimister & Miller, 2015). Because it is already approved by the FDA this therapeutic is available and may be an interesting candidate.
A similar study also found that the cathelicidin produced by adipocytes is different to the type secreted by other cells (Zhang et al., 2015). Further research into these differences may yet reveal alterations in function which could provide insight into its antimicrobial activities and how best to capitalize on its use in therapeutics.
Phimister, E. G., & Miller, L. S. (2015). Clinical Implications of Basic Research: Adipocytes Armed against Staphylococcus aureus.The New England Journal Of Medicine, 3721368-1370.
Zhang LJ, Guerrero-Juarez CF, Hata T, et al. (2015). Innate immunity: dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science, 347:67-71.
@ M.Chreech, drainage usually occurs when S. aureus causes an abscess or boil full of dead leukocytes or puss. Often times S. aureus can cause necrosis of tissue by releasing its toxins such as alpha toxin which induces lysis of various cells and apoptosis of T-lymphocytes (1). An infection with S.aureus can induce the release of pro-inflammatory cytokines such as TNF-α and interleukin-1β causing tissue damage (2). In both the release of pro-inflammatory cytokines and alpha toxin tissue damage can lead to necrosis and the need for surgical debridement of necrotic tissue, in such cases the use of cathelicidin from adipocytes would be ineffective. I’m somewhat skeptical of the powers in increasing the production of cathelicidin from adipocytes to kill S. aureus especially in cases where S. aureus has caused severe tissue damage. In addition, S. aureus could develop a genetic mutation that makes it resistant to cathelicidin.
1. Thay B, Wai SN, Oscarsson J. Staphylococcus aureus α-Toxin-Dependent Induction of Host Cell Death by Membrane-Derived Vesicles. Otto M, ed. PLoS ONE. 2013;8(1):e54661. doi:10.1371/journal.pone.0054661.
2. Wesson CA, Deringer J, Liou LE, Bayles KW, Bohach GA, Trumble WR. Apoptosis Induced by Staphylococcus aureus in Epithelial Cells Utilizes a Mechanism Involving Caspases 8 and 3. Tuomanen EI, ed. Infection and Immunity. 2000;68(5):2998-3001.
@ Dessica Hodges, a recent study in Plos One elucidated the mechanism by which S. aureus resists cathelicidin. S. aureus is mostly resistant to penicillin today, and the newer MRSA strain is resistant to methicillin. S. aureus gains resistance to penicillin by producing beta-lactamases, which are enzymes that break down beta-lactam antibiotics (like penicillin). The gene to make beta-lactamase is suppressed by another gene called BlaI, which provides resistance to cathelicidin. So, BlaI can actually be target for therapy against MRSA. With drugs that target BlaI, MRSA will probably be more susceptible to cathelicidin. A combined therapy of drugs that target BlaI and also increase the ability of adipocytes to make cathelicidin might be the next big victory against MRSA.
Source:
Pence MA, Haste NM, Meharena HS, Olson J, Gallo RJ, Nizet V, Kristian SA. Beta-Lactamase Repressor BlaI Modulates Staphylococcus aureus Cathelicidin Antimicrobial Peptide Resistance and Virulence. PLoS ONE 10(8): August 25, 2015.
Many underestimate how important your skin is in protecting your body from pathogens such as methicillin-resistant Staphylococcus aureus (MRSA). Everyday individuals unnecessarily break this protective barrier when getting tattoos, piercings, cosmetic procedures and surgeries. Unknowingly, inviting pathogens such as S. aureus to invade the body and internal organs, doing tissue damage that is beyond what your immune system can repair including the adipocytes mechanism of killing bacteria by releasing cathelicidin. Once S. aureus has defeated the local innate immune response in the skin and has spread to the blood, causing bacteremia it can lead to sepsis, pneumonia and even worse an infection of the heart called endocarditis. In the heart, S. aureus can cause damage by attaching to myocardiocytes forming vegetations on the heart valves. These vegetations form a bacteria, platelets and plasma protein coagulation (3). This coagulated mass attracts neutrophils and monocytes that release cytokines and procoagulant factors causing the vegetation to enlarge (1, 2). Endocarditis from S. aureus can cause blockages that can lead to myocardial infarction and leaky valves. Once antibiotics are given the enlarged vegetated mass breaks apart and can float to the brain causing strokes. Unfortunately, this happen to my sister and she passed away due to endocarditis caused by S. aureus that invaded her body after a cosmetic procedure.
1. Lepidi H, Casalta JP, Fournier PE, Habib G, Collart F, Raoult D.Quantitative histological examination of bioprosthetic heart valves. Clin Infect Dis. 2006 Mar 1; 42(5):590-6.
2. Veltrop MH, Bancsi MJ, Bertina RM, Thompson J. Role of monocytes in experimental Staphylococcus aureus endocarditis. Infect Immun. 2000 Aug; 68(8):4818-21.
3. Mylonakis E, Calderwood SB. Review Infective endocarditis in adults. N Engl J Med. 2001 Nov 1; 345(18):1318-30.
I completely accept with your argument and concern of damaging the largest protective layer of body. Apart from inviting pathogens while getting tattooed, the ink used in tattooing are also found to cause cutaneous pseudo lymphoma. Cutaneous pseudo lymphoma is one of the potential complications in tattooing.
The article ‘Cutaneous Pseudo lymphoma Following Tattoo Application: Report of Two New Cases of a Potential Lymphoma Mimicker’ discusses two cases who suffered from the symptoms of cutaneous pseudo lymphoma. In both the cases delayed lesions was reported in the areas where red ink was used. The delayed responses are related to the metals and azo compounds, which are the basic components of tattoo inks. Lymphoid infiltration was seen in the upper and middle part of the dermis layer. Scattered macrophages along with ink particles were also noted. Similarly CD 4 T cells and T cells markers like CD 2 ,CD 3, CD 4, CD 5, CD 8 along with little amount of CD 20 positive B lymphocytes were noted in both the cases. These markers also lead to a suspicion of malignant lymphoma .
References:
Debora Camilot, MD , Zoran M. Arnez, MD , Boštjan Luzar, MD , Jože Pižem, MD , Borut Žgavec, MD , and Giovanni Falconieri, MD,2012, Cutaneous Pseudolymphoma Following Tattoo Application: Report of Two New Cases of a Potential Lymphoma Mimicker, International Journal of Surgical Pathology 20(3) 311–315, DOI: 10.1177/1066896911425487.
Though the capacity and function of subcutaneous adipocytes in contribute to the barriers that limit the ability of pathogens to spread throughout the body is a recent discovery, the ability of epithelial tissues to secrete antimicrobial peptides has been a well known component of the body’s built-in barriers. That subcutaneous fat, already known to correlate with improved health of nearby tissue and changes in the cytokine microenvironment would directly produce cathelicidin after being induced by what Dr. Miller theorizes is TLR activity is not a surprise. The ability of subcutaneous adipocytes to form an additional layer for the prevention of sepsis is just added evidence that contrary to popular opinion not all forms of fat are harmful.
Given the protective functions subcutaneous fat may serve and considering that through both diet and aging it and its protection are gradually lost; some form of adipogenic pharmacological therapy has been suggested as a means to improve immunocompetence and overall health. Thiazolidinediones acting as PPAR-γ agonists have been suggested as a possible means to induce subcutaneous adipogenesis by Dr. Miller, however PPAR-γ receptors are present throughout the body and will alter expression, metabolism and even innate immune function. Therefore systemic PPAR-γ activation will not only lead to the development of visceral fat that is known to induce inflammation and be detrimental to health, but also an unacceptable cardiovascular risk. Replicating the effects of infection with TLR ligands would also not be an option in human patients due to the potential for inflammatory damage. Instead, any attempt to promote adipogenesis via signal transduction will either have to work through PPAR-γ partial agonists with affinity in favor of the adipocyte PPAR-γ isoforms or through a more detailed study of the signal transduction cascade that follows from activation of subcutaneous adipocyte TLR receptors. Recent studies have characterized the transcription factor recruitment and cytokine release the activation of adipocyte TLR induces. With a better understanding of immune related adipocyte signal transduction, it may be possible pharmacologically potentiate signals inducing antimicrobial and adipogenic functions within adipocytes.
Bibliography: 1. Nissen S, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. New England Journal of Medicine. 2007;356(24):2457-2471. doi:10.1056/nejmoa072761.
Bibliography: 2. Hamdy O e. Metabolic obesity: the paradox between visceral and subcutaneous fat. – PubMed – NCBI. Ncbinlmnihgov. 2015. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18220642. Accessed November 19, 2015.
Bibliography: 3. Kopp A e. Innate immunity and adipocyte function: ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine sec… – PubMed – NCBI. Ncbinlmnihgov. 2015. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19148127. Accessed November 19, 2015.
Contrary to popular belief the adipocytes are no longer considered as mere energy reserves. They have been recognized for their role in metabolic regulation, neuroendocrine functions and as important immune modulators for over a decade now. Some of the products of adipose tissues (Adiponectin, leptin, resistin and visfatin) are being called as adipocytokines as they have been known to regulate many pathological processes.
Adiponectin reduces B-cell lymphopoiesis, T-cell responses, endothelial adhesion molecules, NFκB response and Phagocytosis. Leptins act as pro-inflammatory molecules by increasing the Neutrophil activation, ROS, Chemotaxis, NK-cell function, Lymphopoiesis, Thymocyte survival, T-cell proliferation, TH1 response. Resistin help in increasing the expression of endothelial adhesion molecules (VCAM1 and ICAM1), Visfatin helps to inhibit apoptosis of neutrophils.
Hence, there are could be several alternatives to using cathelicidin. To control an MRSA infection we could stimulate the upregulation of adipocyte leptins that can further increase NK cell function and chemokine expression thus helping to recruit more neutrophils. The neutrophil activity could be enhanced by subcutaneous administration of anti-MRSA antibodies. In dire need, adipocytes may be stimulated to upregulate Visfatin that may help in keeping neutrophils viable for a long time.
Source: Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature reviews. Immunology. 2006;6(10):772-783.
This article methodically and dynamically sheds light on our own bodies natural defenses and its ability to produce certain components, including Cathelicidin, that could potentially play a significant role in fighting MRSA; a bacterial infection that has demonstrated resistance to a broad spectrum of antibiotics including that of vacomycin.
A study by Dr. Komatsuzawa Hitoshi and his colleagues, which comprised of both in vitro and invivo experiments, complements and expands on some of the major points in this article, regarding the production of Cathelicidin and its anti-microbial characteristics. In the article titled “Innate defenses against methicillin-resistant Staphylococcus aureus (MRSA) infection”, Dr. Hitoshi and his colleagues discuss the nature of Cathelicidin and, and other similar anti-microbial peptides, deriving from human mammalian cells, their role in innate immunity, and their ability to kill and even inactivate bacteria through initiating chemokine activity. According to Dr. Hitoshi, only one Cathelicidin can be found in human beings and it has been identified as LL37/hCAP18. This anti-microbial peptide is cationic in nature, and they frequently bind electrostatically to surface molecules, with negatively charged components. Also according to Dr. Hitoshi’s study, the Cathelicidin family constitutes highly conserved regions referred to as cathepsin L inhibitor which are located at the N-terminus, as well as being present in the variable region on the C-terminus. These are the molecular characteristics that contribute to the anti-microbial characteristics of the peptide Cathelicidin.
LL37/hCAP18 has also been observed as being present in other components of the immune system including neutrophils, monocytes, and various tissues including epithelial cells and sweat glands. LL37 has demonstrated significant capabilities to neutralize, kill, or inactivate gram-negative bacteria, gram-positive bacteria, and has even exhibited the ability to fight antibiotic-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Furthermore, LL37/hCAP18 is capable of neutralizing endotoxic activity by way of binding to LPS. Additionally, LL37 can behave as a chemotactic factor for other components of the immune system including neutrophils, monocytes, T cells, and mast cells.
The membrane of the bacteria is made porous after the anti-microbial peptides have been introduced to the surface of the bacteria. The eventual result is the release of the cytoplasmic contents and the death of the bacteria.
Work Cited:
Komatsuzawa, Hitoshi, et al. “Innate defenses against methicillin‐resistant Staphylococcus aureus (MRSA) infection.” The Journal of pathology 208.2 (2006): 249-260.
That’s a very interesting point Adam. I find it very interesting that the anti-microbial peptide cathelicidin is expressed on so many different cell types. As you mentioned, LL37/hCAP18 is expressed on immunological cells, but also epithelial cells and sweat glands. Additionally, the behavior of LL37/hCAP18 as a chemotactic factor is noteworthy.
Our immune system is incredibly redundant. We have multiple cell types that can behave as antigen-presenting cells. We have multiple phagocytic cells, multiple cytotoxic cells, and many ways to activate lymphocytes. While interesting, I don’t find it surprising that adipocytes are also expressing an anti-microbial peptide. In the future, we may find that every organ and tissue system overlaps in ways we wouldn’t have imagined.
For example, it’s been found that chronic inflammation in adipose tissue is observed in those with excessive body fat. This typically leads to cardiovascular mortality, but can also have a deleterious effect on an obese person’s ability to deal with pathogens. For example, obese people have abnormalities in their granulocytes and Tregs in their adipose tissue due to chronic inflammation.
We’re finding that every cell type in our body interacts with other cell types in complex ways that we’re only beginning to understand. The more we learn, the more extraordinary our bodies seem!
Reference:
Ghigliotti G, et al. Adipose Tissue Immune Response: Novel Triggers and Consequences for Chronic Inflammatory Conditions. Inflammation. 2014; 37(4): 1337–1353
I find this article interesting and useful due to its informative nature and stereotype breaking message. Adipocytes have always been known to be important for energy storage in our cells. However, society and media have long criticized the presence of fat in humans and downplayed its necessity. Studies have been showing for years now that adipocytes are actually more important than ever imagined as they play an understated role in immune system regulation. This occurs notably through release of cathelicidin which can kill bacteria head on or it can release cytokines that recruit the appropriate additional immune cells to better respond to infection. Despite the fact that neutrophils and macrophages also secrete this peptide, I believe having the extra release from adipocytes is critical in supporting immune defense from infection. That is why the experiments yielding higher rates of infection in hosts were typically associated with a deficiency in cathelicidin and also suppression of adipocyte growth. I also believe that utilization of adipocytes in therapy should be adopted however this should be done cautiously considering the ability of pathogens like S. aureus to use cathelicidin to its own advantage to enhance infection. Additionally, S. aureus is one of how many different strains of microbe that have this ability to manipulate the effects of cathelicidin? How does S. aureus’s potency compare to these others and can a standard treatment be applied to all strains? This amount and potency questions must be considered and assessed during the development of vaccines and treatments. The major question remains is if this therapy can be targeted specifically to the desired cell type upon further research. If so there is no telling how effective this can be in preventing the spread of disease that started from infection of the skin. Experimentation with vitamin D has been studied and shown to affect both the innate and adaptive immune systems through regulation of the expression and release of cathelicidin in response to infection. Further analysis with vitamin D will significantly reveal if it is feasible for cathelicidin to be used by the body solely for the benefit of the host and not the pathogen or if exploitation is just going to have to be an undesired consequence of its use. Such findings will undoubtedly lead to groundbreaking discoveries and breakthroughs in therapeutic strategy.
Wei, R., & Christakos, S. (2015). Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Nutrients, 7(10), 8251-8260. doi:10.3390/nu7105392
Leptin is one of the important hormone secreted by adipocytes. Although it is found to plays an important role in maintaining body weight through food intake, it is also found to regulate various innate and adaptive immune responses.
In macrophages leptin regulates phagocytic function through phospholipase action of pro inflammatory cytokines TNF-α, IL-6 and IL-12. Leptin also induces chemotaxis of neutrophils and production of free radicals. Leptin induces activation of markers in both CD4 and CD8 T lymphocytes. It promotes secretion of IL-2 in naïve T cells, on memory T cells. Thereby increasing INF-γ and TNF-α secretion, which increases production of B cells and promoting delayed type hypersensitivity reactions. Leptin also affects the generation, maturation, and survival of thymic T cells by decreasing their rate of apoptosis.
Reference:
Claudio Procaccini, Valentina Pucino, Christos S. Mantzoros, Giuseppe Matarese, Leptin in autoimmune diseases, Metabolism. 2015 Jan;64(1):92-104. doi: 10.1016/j.metabol.2014.10.014.
Skin carries a complex and unique combinations of immune system that help in protecting us from various pathogens and infections. Adipose tissues are located primarily underneath the skin but they are also found surrounding the internal organs where they act as protective layer. Adipocytes play a role in regulation of immunity and inflammation. Macrophages are present in adipose tissues and actively participate in immune regulation. They are increased in obesity. Different types of pro-inflammatory and anti- inflammatory factors are produced by adipose tissues such as adipokines liptin, adiponectin, resistin, visfatin, cytokines and chemokines (Tumor Necrosis Factor- α, IL-6, Monocytes chemoattractant protein 1, and others).
These pro-inflammatory molecules actively take part in insulin resistance and increased risk of cardiovascular diseases. It is shown that reduced leptin level can increase chances of infection due to reduced T cell response in under nourished individuals. A variety of inflammatory conditions leads to change in adipokine level.
Adipose tissues are of two types; white adipose tissues (WAT) and brown adipose tissues. Circulating levels of TNF-α and IL-6 are directly correlated with adiposity and insulin resistance. Macrophages are the major source of TNF-α produced by WAT and contribute approximately 50% of WAT-derived IL-6.
References:
Ilkovitch D. Role of immune-regulatory cells in skin pathology. J Leukoc Biol.2011 Jan;89(1):41-9. doi: 10.1189/jlb.0410229. Epub 2010 Jul 13. Review.
http://www.sciencedirect.com/science/article/pii/S0091674905004173
Adipocytes are known to be immunologically active and to have a role in the defense mechanisms; however, the exact role is unclear. The present adipocytes proliferate in the case of infection and when these cells are absent, there is an increase in infection. Adipocytes were not, until recently, known as a part of the skin defense, they were known for their role of storing fat as energy. Understanding that these cells are a part of the population, secreting cathelicidin in response to invading pathogens, such as, MRSA is enough to prove the importance of these cells. Infections caused by bacteria, such as, MRSA and their ability to thrive could be pinned to the inhibition of adipocytes or some type of malfunction within these cells. The adipocytes are found in the skin (dermis) and this is where most bacteria seek to enter the body. The immune system is such a mechanistic system that bacteria should lose the battle when invading and when there is room for the bacteria, it is safe to say that there is some type of immunological malfunction. There should be more studies focusing on the defense team found in the skin, since this is one of the most neglected organs of the body and it’s role in immune response is quite underestimated.
Actually studies have shown that adipose tissue secrete adipokines, hormones that send messages to immune cells as well as other parts of the body to communicate nutrient availability. As such they regulate many functions within the body, including the immune response which requires a large amount of energy to function. When there is insufficient nutrients (i.e. starvation or otherwise inadequate amount of fat within the body) there is a limited immune response to infection both in proliferation as well as activity due to the lack of availability of energy. On the opposite spectrum, when there is an overabundance of fat, the immune system becomes overactive within adipose tissue and leads to chronic inflammation as well as other immune disorders.
Wensveen, F. M., Valentić, S., Šestan, M., Turk Wensveen, T., & Polić, B. (2015). Interactions between adipose tissue and the immune system in health and malnutrition. Seminars In Immunology, doi:10.1016/j.smim.2015.10.006
While this article made me feel somewhat less horrified by my Thanksgiving feasting (knowing that my growing adipocytes are likely giving my immune system a boost), what I found most intriguing was the experimental design choice that the researchers used. Watanabe et. al. first grafted human foreskin (from circumcisions of newborns) onto nude mice, then administered intravenous adult human blood, triggering an adaptive immune response with formation of memory T cells. They then treated the mice with alemtuzumab to distinguish resident from recirculating T cells, subsequently confirming the existence of four distinct subpopulations of memory T cells.
Alemtuzumab is a recombinant DNA-derived humanized monoclonal antibody that binds to CD52, a glycoprotein antigen present on the surface of malignant and normal, mature B and T cells (as well as on monocytes, NK cells and macrophages), where it triggers ADCC (antibody-dependent cellular cytolysis) and complement-mediated lysis (Coles et. al., 1999). The FDA has approved alemtuzumab for the treatment of B-cell chronic lymphocytic leukemia, cutaneous T-cell lymphoma, and highly-active, relapsing-remitting MS, as well as immunosuppressive therapy following certain organ transplants, but it has also been used to treat several other autoimmune disorders (Fernandez, 2014). Its primary mechanism of action is by altering the number, proportion and properties of some lymphocyte subsets, effectively rebooting the immune system.
Utilizing alemtuzumab to specifically deplete recirculating T cells from skin in order to analyze the remaining resident T cells was an ingenious experimental method. All nonrecirculating resident memory T cells expressed CD69, with two distinct classes identified by the expression or absence of cell surface marker CD103. Those in the outer epidermis expressed CD103 and produced great quantities of cytokines, while CD103- cells resided in the dermis and proliferated more, but with lower cytokine production. Both the resident memory T cells demonstrated stronger innate effector functions than recirculating T cells, which were CCR7+/L-selectin+ or – (central memory TCM or migrating memory TMM). Both recirculating memory T cells induced distinct inflammatory skin lesions, with TMM recirculating more slowly and serving as cytokine intermediates between TCM and effector memory T cells. This study provided an amazing advancement in the understanding of the human immune system, with the creative usage of foreskin and an antineoplastic drug, and may prompt new therapeutic delivery mechanisms for immune and autoimmune disorders, as well as other neoplasms.
Coles AJ, Wing MG, Molyneux P, et al. 1999. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. (Online), 46 (3), 296–304. PMC. http://www.ncbi.nlm.nih.gov/pubmed/10482259 (accessed Nov. 27, 2015).
Fernandez, Óscar. 2014. Alemtuzumab in the Treatment of Multiple Sclerosis. Journal of Inflammation Research 7 (Online), 19–27. PMC. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3959804/#b1-jir-7-019 (accessed Nov. 27, 2015).
Watanabe, R. et. al. 2015. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Science Translational Medicine (Online), 7 (279), 279-290. http://stm.sciencemag.org/content/7/279/279ra39.full (accessed Nov. 27, 2015).
The first time I took Immunology during undergrad, the importance of the skin and its role in the immune system could not be stressed enough. Therefore, I’m not completely surprised about the immune role of adipocytes. What caught my eye most was the role of the active form of Vitamin D in cathelicidin expression. Another study in Nutrients Magazine showed that activating Toll-like receptors (TLRs) on monocytes, in combination with Vitamin D, stimulates the expression of cathelicidin. The article also states that keratinocytes, which are located in the epithelium, Vitamin D increases TLRs and cathelicidin expression [1]. There has been increasing studies that prove how beneficial Vitamin D is to our immune system. Unfortunately, many insurance companies do not take note of this information and choose not to cover testing that looks for Vitamin D deficiency. After suffering from recurrent infections myself, my doctor decided to check my Vitamin D levels. It was at 19 ng/ml when the preferred range is a minimum of 31 ng/ml. After receiving intensive Vitamin D therapy, I haven’t had as many recurring infections.
Works cited:
Mechanisms Underlying the Regulation of Innate and Adaptive Immunity by Vitamin D. Ran Wei and Sylvia Christakos. Nutrients. 2015 Oct 7 (10): 8251-8260.